Controlling CO2 levels in laparoscopic surgery
Low flow, low pressure surgery
A review of how the technology that Ultravision employs, enables one of the key elements for ‘best practice’ laparoscopic surgery of establishing pneumoperitoneum.
Laparoscopic surgery is increasingly becoming the standard of care for many abdominal procedures[i]. Regardless of the complexity, all procedures have four essential requirements in common:
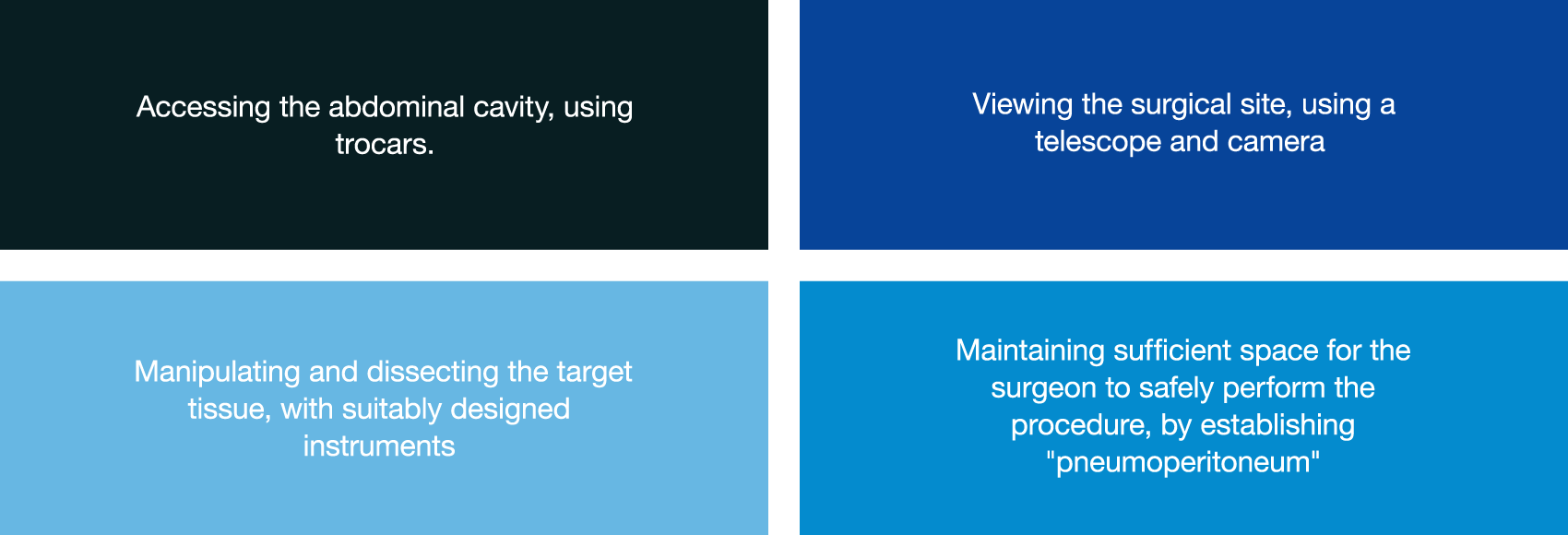
In recent years there have been striking advancements in access, visualization and instrumentation that have enabled increasingly complex procedures to be performed. However, there has been relatively little innovation in the means of establishing and maintaining pneumoperitoneum.
Achieving pneumoperitoneum
There are two ways to provide the surgeon with sufficient working space inside the abdomen to safely perform a laparoscopic procedure. The first – manually separating the abdominal wall from the abdominal contents using physical “lifting” – has not been widely adopted because of surgeons’ concerns about the operative field of view and damage to the abdominal wall during the lift[ii]. Accordingly, the second – using pressurised gas to lift the abdominal wall away from organs – is the standard of care globally. Gas is introduced into the abdomen by an insufflator via tubing that (after initial entry) is attached to the trocars used to introduce instruments into the abdomen.
Candidate insufflation gases used to establish pneumoperitoneum must be cheap, colourless, non-flammable, inexplosive, easily removed by the body, and non-toxic to patients and operating room staff. Inert gases such as helium, nitrous oxide, and carbon dioxide (CO2) have been evaluated, with CO2 becoming used almost ubiquitously.
CO2 – less is more
Although CO2 meets most of these requirements it is by no means perfect. There are four aspects to consider in assessing its patient impact on both local and systemic tissues and organs during surgery:
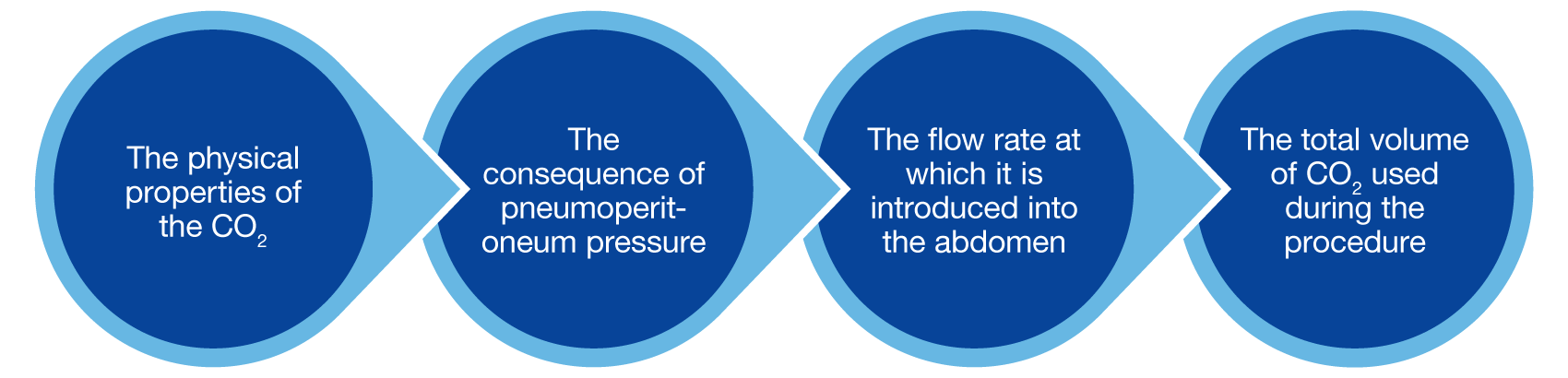
CO2 supplied from tanks or a wall supply in the OR is bone-dry (0% relative humidity) and very cold (~20C, 68F), which is ~16C lower than body temperature. Patient CO2 exposure during laparoscopic surgery has both local and systemic implications, including:
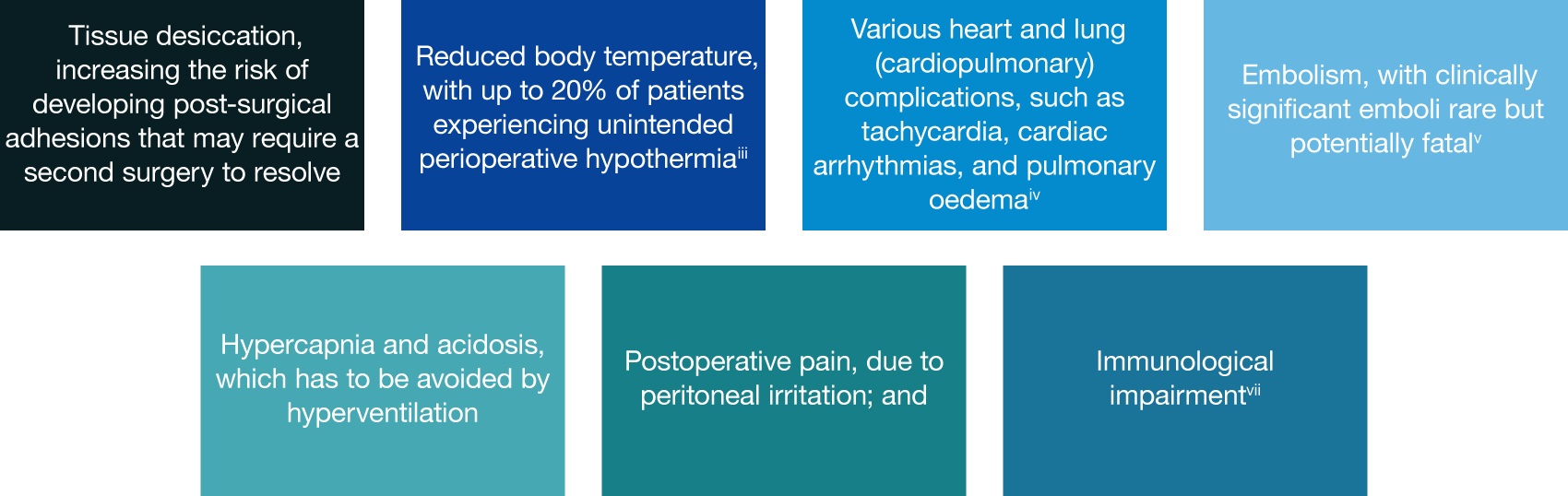
Systems have been developed that serve to heat and humidify the CO2 before it is introduced into the patient. However, an independent Cochrane review has demonstrated that the clinical benefits of heating and humidifying CO2 remain unclear.[iii]
The pneumoperitoneum pressure has also been shown to influence patient outcomes. Pressures of 15mmHg or more have historically been used to ensure sufficient operative space is available throughout a procedure. This pressure also allows for the removal of CO2 by suction – to remove surgical smoke or fluids – without complete loss of the space. However, low pneumoperitoneum pressure (typically 10mmHg or less) has been demonstrated to improve patient outcomes in upper GI, gynaecology, and colorectal surgery[iv]. Recent concerns over the potential for surgical smoke to contain infectious biological agents have also led many surgical societies to recommend reducing pneumoperitoneum pressure to minimize the ejection force of accidental or intentional leaks during surgery, thereby reducing its transmissibility[v].
“I think that [low pressure surgery] is a big advantage in terms of pain management because the patient does not have the over-distention of the peritoneum, which causes pain.” – Mr Gourab Misra
The need to rapidly and frequently remove CO2 from the abdomen to clear the visual field has also led to the development of “high flow insufflators” that can introduce gas at a rate of up to 50 litres per minute, almost a litre per second. However, the effectiveness of these is limited largely by the size of the aperture through which the gas is introduced. Furthermore, high gas flow rate has also been demonstrated to be associated with increased risk of cardiovascular complications and post-operative pain[vi].
The average volume of CO2 required to achieve pneumoperitoneum is typically around 3 litres[vii]. However, gas consumption vastly exceeds this. Although continued CO2 absorption and excretion accounts for some of this volume, the majority of CO2 is consumed because of the ongoing removal and exchange to maintain a clear view of the operative site. As a result, volumes often exceed 100 litres per hour of surgery, with some longer, complex cases exceeding a thousand litres in total. Recent work has also highlighted the extent to which CO2 is continually lost during surgery because of accidental leaks that occur around and through trocars; during instrument exchanges and specimen removal[viii]; and through hollow instruments[ix]. This hugely excessive CO2 exposure exacerbates the issues listed above.
Best practice for 21st Century surgery
Recognising the limitations of gas-less laparoscopy, the excessive use of CO2, and the associated complications this causes during and after surgery, Alesi Surgical developed Ultravision. Using the proven process of electrostatic precipitation, Ultravision is the only technology that can provide a clear visual field throughout surgery and prevent the need to vent surgical smoke into the operating theatre without requiring ongoing CO2 exchange[x]. This lack of gas exchange provides the surgeon with stable pneumoperitoneum and the opportunity to reduce both pneumoperitoneum pressure and rate of gas exchange from the insufflator. The advantages to the surgeon, the perioperative team and the patient are compelling.
[i] The benefits of shorter patient recovery time and reduced duration of stay have proven that, where appropriate, laparoscopic surgery should be the preferred option.
[ii] Ren et al https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4100966/
[iii] Birch et al
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD007821.pub3/pdf/full
[iv] Colorectal: Celarier et al (https://pubmed.ncbi.nlm.nih.gov/33755088/); Gynaecology: Radosa et al (https://pubmed.ncbi.nlm.nih.gov/31136069/); Upper GI: Hua et al (https://pubmed.ncbi.nlm.nih.gov/24503370/)
[v] SAGES/ EAES recommendations for surgery during Cov-19 pandemic 2020
https://www.sages.org/recommendations-surgical-response-covid-19/
[vi] Berberoglu et al https://www.liebertpub.com/doi/pdf/10.1089/lap.1998.8.273 and Jung et al https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3790044/
[vii] Phillips et al https://onlinelibrary.wiley.com/doi/abs/10.1046/j.1365-2508.1999.00342.x
[viii] Hardy et al
https://pubmed.ncbi.nlm.nih.gov/33829231/
[ix] Dalli et al
https://pubmed.ncbi.nlm.nih.gov/32691961/
[x] Ansell et al
https://pubmed.ncbi.nlm.nih.gov/24570011/;
Levine et al
https://pubmed.ncbi.nlm.nih.gov/33100818/.
